Grade 8 Science: Metal Crown
Grade 8 Science: Metal Crown
1: Density of Metal Block
Correct Responses
-
19.2 g/cm3
Note: Extra trailing zeroes may also be added (e.g., 19.20, 19.200)
- 19 g/cm3 [Rounds to nearest whole unit.]
Incorrect Responses
-
Shows the set-up for density (mass/volume) but does not compute density
or makes a computational error.
-
125 [Computes volume but not density.]
-
19.3 [No work shown; indicates density copied from table.]
- Other incorrect
Grade 8 Science: Metal Crown
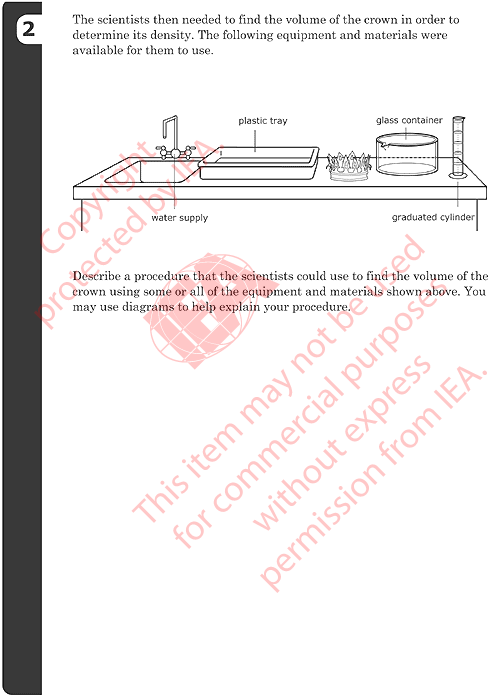
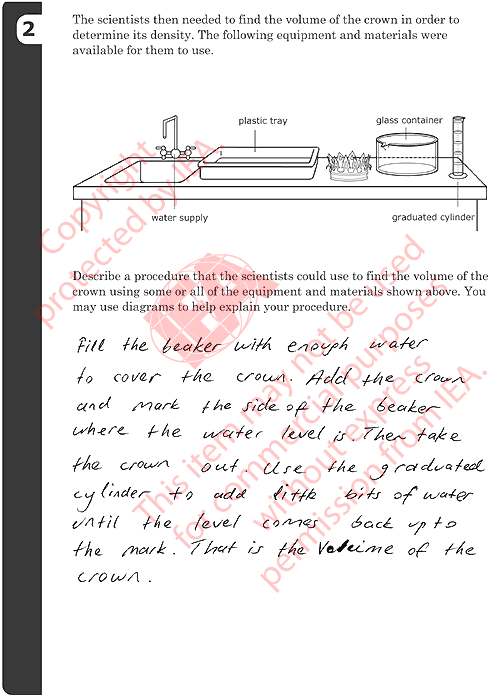
2: Procedure to Find Volume of Crown
Correct Responses
- Describes or diagrams a procedure based on displacement of water using measured water level differences:
- i) Adding water to the beaker (sink or tray) and marking the water
level.
- ii) Placing the crown in the beaker (sink or tray) and marking the
new water level.
- iii) Measuring the volume difference before/after adding the crown
using the graduated cylinder
- Describes or diagrams a procedure based on displacement of water using
measured overflow:
- i) Filling the beaker (or tray) with water.
- ii) Placing the crown in the beaker (or tray) and collecting the overflow.
- iii) Measuring the volume of the overflow using the graduated cylinder
Partial Response
-
Describes or diagrams a partial procedure that includes displacement
of water but with inadequate or no description of the steps/measurements
to determine the volume.
- Examples:
- Put some water in the beaker and add the crown. Measure how
much the level of water went up.
- Add the crown to the beaker filled with water. See how much overflowed.
Incorrect Responses
-
Mentions putting the crown in the beaker (sink or tray) of water with
no explicit mention that the water level will rise/overflow and
no or incorrect procedure given for measuring the volume.
- Examples:
- Fill the beaker to the top with water and add the crown. You can get the volume that way.
- Other incorrect
Grade 8 Science: Metal Crown
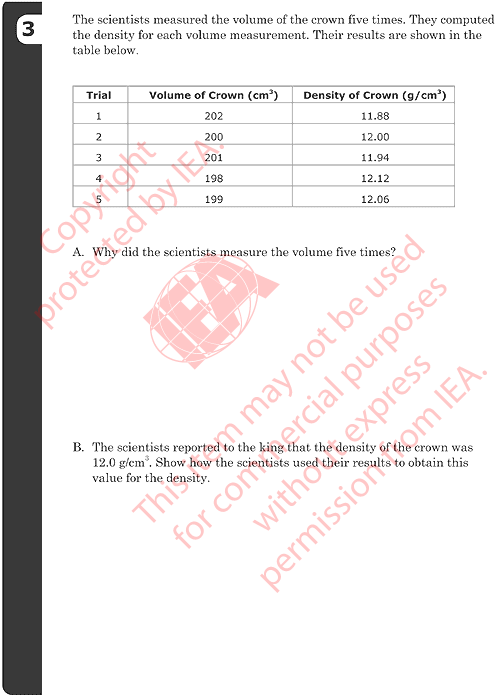
3A: Why Scientists Repeat Measurements
Correct Responses
-
Refers to accuracy, precision, reliability, experimental uncertainty,
estimation of measurement error (or similar).
- Examples:
- It’s more reliable.
- Each time they measure the volume it is close but not exactly the same. So, it’s better to measure it a few times to be sure.
- They want a more exact answer.
- To get an accurate measure of the volume.
-
Refers only to computing an average or mean value (or median or range).
- Examples:
- To find the average volume.
- To work out the mean.
Incorrect Responses
-
Refers only to ‘mistakes’ or changes in the measurements (or
similar); no explicit mention of accuracy, precision, experimental
uncertainty, etc.
- Examples:
- In case mistakes happen.
- To make sure it wasn’t changing.
- To make sure the answer was right and he did not make
a mistake.
- To make sure they did it right.
- To check if it was correct.
- Refers only to a ‘fair test’ or similar; no explicit
mention of computation of average, accuracy, precision, experimental
uncertainty,
etc.
- Examples:
- To make sure it was a fair test.
- To ensure a fair test.
- Other incorrect
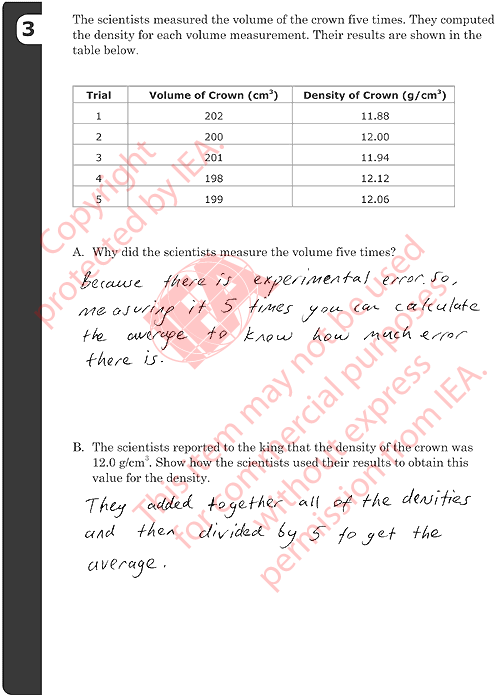
3B: Obtaining Average (Median) Value
Correct Responses
-
Shows (or describes) a correct method for computing the average (mean)
value.
- Examples:
- (11.88+12.00+11.94+12.12+12.06) = 60. 60/5=12.0
- (202+200+201+198+199)/5 = 200. 2400/200=12.0
- They added together all of the densities and then divided by 5 to
get the average.
-
Shows (or describes) a correct method for determining the median value.
- Examples:
- 202, 201, 200, 198, 199. 200 is the median volume, so 2400/200
is the median density (12).
- 12 is the middle value when placed in order (12.12, 12.06, 12.00,
11.94, 11.88).
Incorrect Responses
-
States that it is the average, mean or median value with no or incorrect
work shown.
-
Shows a computation of density (mass/volume). [No determination of
average or median included.]
- Examples:
- They did mass divided by volume.
- 2400g/200cc = 12 g/cc
- Other incorrect
Grade 8 Science: Metal Crown
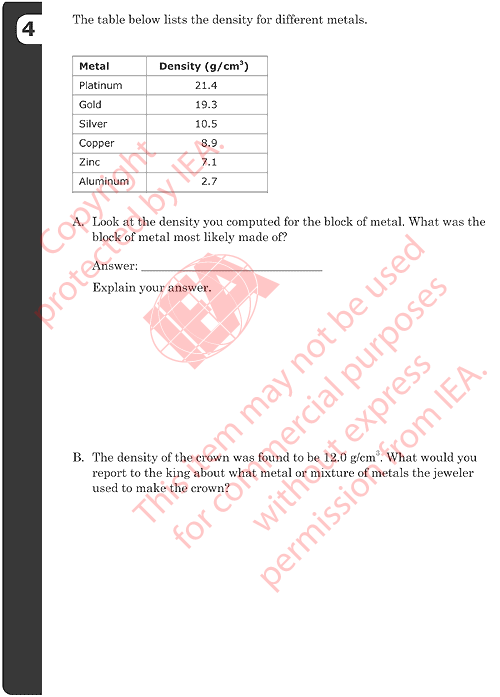
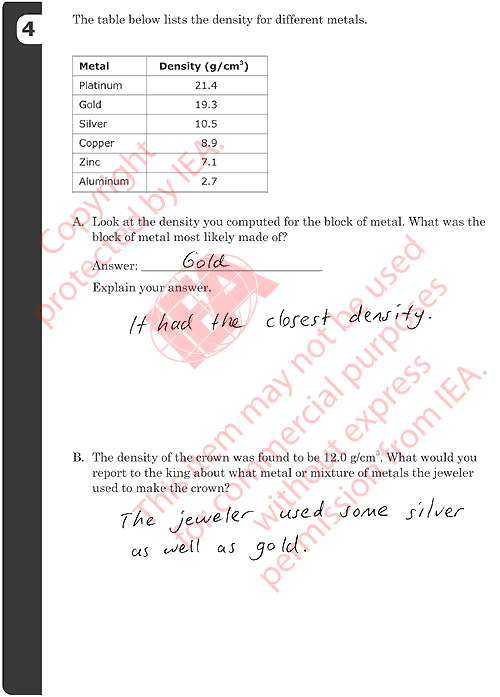
4A: Identifying Metal in Block
Correct Response
-
GOLD with an explanation based on correct density computed in previous
question (19.2 g/cm3).
- Examples:
- Gold. The density is the same.
Incorrect Responses
-
GOLD with no explanation or incorrect explanation that is NOT based
on density.
- Examples:
- Gold. Because that is what crowns are always made of.
-
SILVER (alone or mixed). [Confuses density of crown with density of
the metal block.]
- Examples:
- It is mostly silver because the density is 12 and that’s
the closest one.
- Other incorrect
4B: Reporting Composition of Crown
Correct Response
-
Reports that the crown is made of a mixture (alloy) AND names specific
metal(s) that might be included (reasonable composition based on density).
- Examples:
- The jeweler used some silver as well as gold.
- It might have had some copper mixed in because that would lower the
density and the cost.
- The jeweler most likely used all silver except for a thin coat of
gold to make it look pure gold even though it wasn’t.
Incorrect Responses
-
Reports only that the crown is made of a mixture or is NOT pure gold
(or similar); NO specific metals are named.
- Examples:
- The jeweler didn’t use the block of metal that the king gave
him.
- The jeweler used four more metals to make the crown.
-
Reports SILVER (density closest to 12 g/cm3).
- Examples:
- The metal used is silver.
-
Reports an incorrect mixture of metals based on additive densities.
- Examples:
- It’s silver and aluminum (10.5 + 2.7)
- Mixture of silver and aluminum as their density adds up to 12.0 approximately.
- Copper and aluminum.
- Other incorrect
|
|
© 2008 International Association for the Evaluation of Educational Achievement (IEA)